Melatonin, a pineal hormone, is secreted following a diurnal rhythm or circadian cycle. The cyclical secretion of this amine derivative has many different physiological effects. In this blog, the structure, origin, regulation, and function of melatonin will be explored.
 Figure 1: The chemical structure of N-acetyl-5-methoxytryptophan, or melatonin. (from: http://www.hbcprotocols.com/sleep/images/melatonin.gif)
Figure 1: The chemical structure of N-acetyl-5-methoxytryptophan, or melatonin. (from: http://www.hbcprotocols.com/sleep/images/melatonin.gif)Structure & Secretion
Melatonin, N-acetyl-5-methoxytrptophan, is an indoleamine; an amine derivative. Amine derivitives are relatively small molecules; melatonin having a molecular weight of 232. This hormone is produced from enzymatic processing of the amino acid tryptophan (Hadley & Levine, 2007); see "Biosynthesis" section below.
Melatonin is:
- water-soluble
- synthesized in the cytoplasm of pinealocytes
- stored in secretory vesicles
- released by exocytosis
- transported in the blood as a dissolved molecules
- quickly degraded (half-life of minutes)
- bound by receptors found on the surface of target cell membranes
- capable of activating a second messenger system within the target cell and changing the activity of existing proteins.
Melatonin can be found circulating in the blood plasma and urine of all animal, demonstrating the diurnal pattern:
- levels highest at mid-night (or mid-dark) periods
- levels lowest at mid-day (or mid-light) periods
Organ of Secretion Figure 2: Position of pineal gland, and suprachiasmatic nucleus, within the human brain (from: http://history.wisc.edu/sommerville/351/351images/pineal.jpg)
Figure 2: Position of pineal gland, and suprachiasmatic nucleus, within the human brain (from: http://history.wisc.edu/sommerville/351/351images/pineal.jpg)
 Figure 2: Position of pineal gland, and suprachiasmatic nucleus, within the human brain (from: http://history.wisc.edu/sommerville/351/351images/pineal.jpg)
Figure 2: Position of pineal gland, and suprachiasmatic nucleus, within the human brain (from: http://history.wisc.edu/sommerville/351/351images/pineal.jpg)Melatonin is produced by the pineal gland; a small pine-cone-shaped organ formed by evagination of the diencephalon roof during embryonic development (Hadley & Levine, 2007). It is part of the epithalamus lies deep within the brain, in the posterior portion of the roof of the third ventricle. It contains specialized secretory cells called pinealocytes, neurons, and neuroglia, mainly astrocytes (Martini, 2006). Unmylinated axons of nerve fibers form synapses with the pinealocytes, their synaptic vesicles containing norepinephrine. Serotonin is also found in the sympathetic nerve terminals, as well as the pinealocytes (Junqueira & Carneiro, 2005). Figure 3: Histological section of the pineal gland and different cell types (from: http://w3.ouhsc.edu/histology/Glass%20slides/41_02.jpg)
Figure 3: Histological section of the pineal gland and different cell types (from: http://w3.ouhsc.edu/histology/Glass%20slides/41_02.jpg)
 Figure 3: Histological section of the pineal gland and different cell types (from: http://w3.ouhsc.edu/histology/Glass%20slides/41_02.jpg)
Figure 3: Histological section of the pineal gland and different cell types (from: http://w3.ouhsc.edu/histology/Glass%20slides/41_02.jpg)The pineal gland is said to be a neuroendocrine transducer in which a neural input is transduced into a chemical signal. The pineal gland act as an intermediate between external conditions (photoperiod, as described below) and internal secretion of melatonin (Hadley & Levine, 2007).
Biosynthesis
When light is depleted, the amino acid tryptophan is converted to 5-hydroxytryptophan by the enzyme tryptophan hydroxylase. 5-hydroxytryptophan is is then converted to 5-hydroxytryptamine, or serotonin, by the enyzyme 5-hydroxytryptophan decarboxylase. The enzyme N-acetyltransferase converts serotonin to N-acetylserotonin, which is then O-methylated by by hydroxyindole-O-methyltransferase to finally produce N-acetyl-5-methoxytryptamine, or melatonin (Hadley & Levine, 2007).
 Figure 4: Biothsynthesis pathway of melatonin in the pineal gland (from: http://scienceblogs.com/clock/upload/2006/11/melsythesis.jpg)
Figure 4: Biothsynthesis pathway of melatonin in the pineal gland (from: http://scienceblogs.com/clock/upload/2006/11/melsythesis.jpg)Degradation of melatonin occurs in the liver, where melatonin hydroxylase converts melatonin to 6-hydroxymelatonin, which can be excreted in the urine (Hadley & Levine, 2007).
Regulation
The locus of control varies among animals. In birds, an internal "clock" is within the pineal gland itself, lending to the rhythmic release of melatonin. In mammals, however, photic cues from the eyes are processed by the suprachiasmatic nucleus (SCN) in the hypothalamus (Hadley & Levine, 2008). Figure 5: The suprachiasmatic nucleus, or SCN (from: http://publications.nigms.nih.gov/thenewgenetics/images/ch3_rythm.jpg)
Figure 5: The suprachiasmatic nucleus, or SCN (from: http://publications.nigms.nih.gov/thenewgenetics/images/ch3_rythm.jpg)
 Figure 5: The suprachiasmatic nucleus, or SCN (from: http://publications.nigms.nih.gov/thenewgenetics/images/ch3_rythm.jpg)
Figure 5: The suprachiasmatic nucleus, or SCN (from: http://publications.nigms.nih.gov/thenewgenetics/images/ch3_rythm.jpg)Light information (presence, intensity, etc.) gathered by the photoreceptors in the retina, is conveyed to the SCN by the retinohypothalamic pathway. The retina is activated in the absence of light, i.e. during the night, or in a dark room, leading to the release of melatonin. Light suppresses the biosynthesis of melatonin during the day or in a well-lit room. Figure 6: Primary structures involved in the pathway of transduction of photic cues to chemical release of melatonin (from: http://biology.ucf.edu/~logiudice/zoo3713/Files/image1156.gif)
Figure 6: Primary structures involved in the pathway of transduction of photic cues to chemical release of melatonin (from: http://biology.ucf.edu/~logiudice/zoo3713/Files/image1156.gif)
 Figure 6: Primary structures involved in the pathway of transduction of photic cues to chemical release of melatonin (from: http://biology.ucf.edu/~logiudice/zoo3713/Files/image1156.gif)
Figure 6: Primary structures involved in the pathway of transduction of photic cues to chemical release of melatonin (from: http://biology.ucf.edu/~logiudice/zoo3713/Files/image1156.gif)Melatonin is synthesized and released in response to norepinephrine released from postganglionic neurons from the superior cervical ganglia. Norepinephrine interacts with beta1-adrenoreceptors on the surface of the pinealocytes to increase pineal cAMP. This increase stimulates the biosynthesis pathway, converting tryptophan to melatonin.
Effects
Melatonin's actions include brain, pituitary, and antigonadal. Melatonin is involved in the regulation of many physiological processes, including:
- stimulatory/inhibitory effects on mammalian reproduction
- immune function
- metabolism
- circadian rhythms
1) Melatonin's Influence on Reproductive Function

EARLY OBSERVATIONS:
Evidence of melatonin's physiological effects on mammalian reproduction was first reported by Heubner. He found that young boys who had tumours which had destroyed the pineal gland had exceptionally early maturation and development. This demonstrates melatonin's antigonadal effects in mammals and explains why it was originally regarded as an antigonadotropic hormone (Hadley & Levine, 2007). Melatonin also slows the maturation of sperm and oocytes (Martini, 2006). Continuous exposure to light or dark can hence have an effect on gonadal development. In rodents, maturation of the gonads is delayed when the animals were kept in the dark; when melatonin levels would be highest. To support this, a pinealectomy (removal of pineal gland), which would remove the source of melatonin secretion, counteracts these effects. On the other hand, continuous light exposure, resulting in low levels melatonin biosynthesis, leads to early sexual development and more rapid growth of the gonads themselves. To further support this, injections of pineal extracts, and synthetic melatonin, slow gonadal maturation. It can therefore be condluded that photic cues received by the animal affects the release of pineal melatonin, which in turn modulates reproductive hormone secretions (Hadley & Levine, 2007).
MODERN-DAY FINDINGS:
It is now known that melatonin inhibits gonadal maturation by reducing the rate of GnRH (gonadotropin releasing hormone) secretion by the pituitary gland (Martini, 2006).
It is also known that melatonin can have both inhibitory AND stimulatory effects on the mammalian reproductive system depending on:
- the context in which it is released
- the pattern of release
- short duration secretions of melatonin during summer nights stimulates the reproductive axis
- long duration secretions of melatonin during winter nights inhibit the reproductive axis
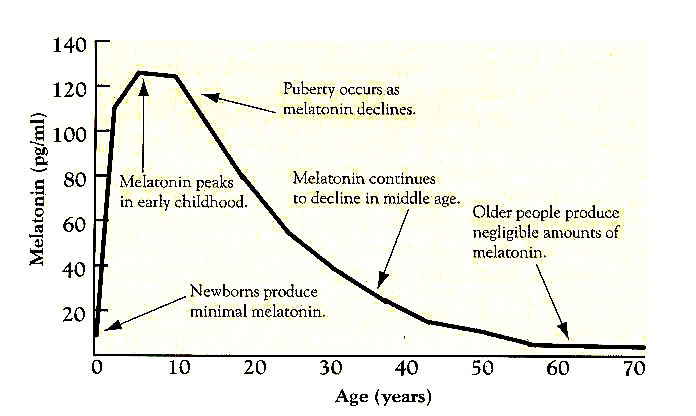 Figure 7: The change in melatonin release throughout the life cycle (from: http://www.benbest.com/nutrceut/mela_age.jpg)
Figure 7: The change in melatonin release throughout the life cycle (from: http://www.benbest.com/nutrceut/mela_age.jpg)2) Melatonin & Immune Function

Recent scientific studies have proposed a link between pineal melatonin secretion and immune function. Pinealectomy, as well as other experimental methods, induce a state of immunodepression. This state is counteracted by injection of melatonin. Hence, melatonin is thought to be an immuno-enhancer (Maestroni, 2007).
The immunoenhancing action of melatonin seems to be mediated by T-helper cell-derived opioid peptides, lymphokines, and perhaps by pituitary hormones. Lymphokines such as gamma-interferon and interleukin-2, as well as thymic hormones, can modulate the synthesis of melatonin in the pineal gland (Maestroni, 2007), representing a negative feedback mechansim.
A main target of melatonin is a primary organ of the immune system; the thymus. Melatonin receptors are found on cells of the immune system, and the binding sites demonstrate diurnal variation in density; i.e. higher densities of receptors found at mid-day when melatonin levels are lowest (Poona et al., 1994).
Melatonin & Metabolism
Melatonin is an effective antioxidant which protects the central nervous system from damage by free radicals such as nitric oxide and hydrogen peroxide (Martini, 2006).
Melatonin oxidation is also important for the production of other metabolites such as N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK). These metabolites also have protective properties (Hardeland & Pandi-Perumal, 2005).

Melatonin & Sleep

Our sleep cycle is greatly influenced by the pulsatile secretion of melatonin following a circadian rhythm. Melatonin is a hormone under control of our internal "biological clock", leading to concentrations highest in the evenings, and lowest during the day (Hadley & Levine, 2007).
Light suppresses the biosynthesis of melatonin during the day or in a well lit room. As night falls, or if we are left in a dark room for some time, the amount of light entering the eye is diminished. It is during this time that melatonin levels are the highest, leading to feelings of sleepiness. This had lead to interest by pharmaceutical companies in search of a more natural treatment for sleep disorders, as well as several other conditions.
 Seasonal changes in the daylight period will also have an affect on melatonin secretion, leading to some interesting changes in the animal. An example of this in humans is SAD; Seasonal Affective Disorder. During the winter months, when days are shorter and light exposure is minimal, some people tend to become lethargic, moody, and depressed due to over-production of melatonin. This is more common at high latitudes, where days are shorter and sunlight is lacking (Martini, 2006).
Seasonal changes in the daylight period will also have an affect on melatonin secretion, leading to some interesting changes in the animal. An example of this in humans is SAD; Seasonal Affective Disorder. During the winter months, when days are shorter and light exposure is minimal, some people tend to become lethargic, moody, and depressed due to over-production of melatonin. This is more common at high latitudes, where days are shorter and sunlight is lacking (Martini, 2006).References:
Hadley, Mac E, and Jon E. Levine. Endocrinology. 6th ed. Upper Saddle River, NJ: Pearson Prentice Hall, 2007.
Hardeland, Rüdiger, and SR Pandi-Perumal. "Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug." Nutrition & Metabolism 2(2005): 1743-7075.
Hill, Richard W, Gordon A. Wyse, and Margaret Anderson. Animal Physiology. 1st ed. Sunderland, MA: Sinauer Associates, 2004.
Junqueira, Luiz C, and Jose Carneiro. Basic Histology: Text and Atlas. 11th ed. USA: The McGraw-Hill Companies, 2005.
Maestroni, Georges J. M. "The immunoneuroendocrine role of melatonin." Journal of Pineal Research 14(2007): 1-10.
Martini, Frederic H. Fundamentals of Anatomy and Physiology. 7th ed. San Francisco, CA: Pearson Benjamin Cummings, 2006.
Poona, Angela M.S., Z.M. Liua, C.S. Pang, G.M. Brown, and S.F. Panga. "Evidence for a Direct Action of Melatonin on the Immune System." Neurosignals. 3(1994): 178-189.